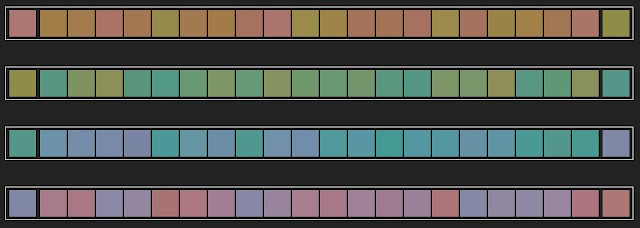
This week for a treat we found a fun quiz from X-rite that tests hue discrimination. It determines which sections of the spectrum you see well and where you have less acuity. Just like golf we are aiming for the lowest score possible.
When color insensitivity gets more severe it turns in to color blindness. But in a species with such amazing evolved traits why would we still be left with 5% (mostly male) of the population with detrimental vision? Wikipedia explains:
"Any recessive genetic characteristic that persists at a level as high as 5% is generally regarded as possibly having some evolutionary advantage over the long term, such as better discrimination of color camouflaged objects especially in low-light conditions. At one time the U.S. Army found that color blind people could spot "camouflage" colors that fooled those with normal color vision. Humans have a higher percentage of color blindness than macaque monkeys according to recent research."
So how did we do on the test? Pretty well actually. We came out with a score of 11 and a mild blue-violet insensitivity.
But we what to know how our color smart readers do!
- Emily Eifler, Writer, Colour Studio
- Jill Pilaroscia, Principal, Colour Studio